STORE INFO
Location & Store Hoursmy cart
Secure checkout
Cannabidiol (CBD), a non-intoxicating compound derived from the cannabis plant, has garnered significant attention for its potential therapeutic benefits across a spectrum of health conditions.
Its versatility has led to a surge in scientific inquiries and clinical trials, aiming to substantiate its effectiveness and safety across various medical contexts. As our understanding deepens, there is hope that CBD could become a cornerstone in treating numerous ailments, providing a natural and effective therapeutic option.
A landmark development in this field occurred when the U.S. Food and Drug Administration (FDA) approved a CBD-based medication for the treatment of specific seizure disorders.
This groundbreaking approval underscores the therapeutic potential of CBD and also paves the way for future research and acceptance of cannabinoid-based treatments in mainstream medicine.
So, what does the science say about CBD and epilepsy?
For years, researchers have explored the potential of CBD for epilepsy, investigating how this non-intoxicating compound interacts with the brain and nervous system.
Early studies and anecdotal reports suggested that CBD might help reduce seizure activity, sparking interest in its therapeutic applications.
As scientific understanding deepened, clinical trials were conducted to evaluate CBD’s safety and efficiency in treating seizure disorders. This research laid the foundation for the first FDA-approved CBD treatment, Epidiolex, marking a significant milestone in epilepsy care.
Unlike taking over-the-counter CBD oil for epilepsy, which lacks regulatory oversight, this prescription treatment has undergone rigorous testing to ensure its reliability for patients with severe and drug-resistant epilepsy.
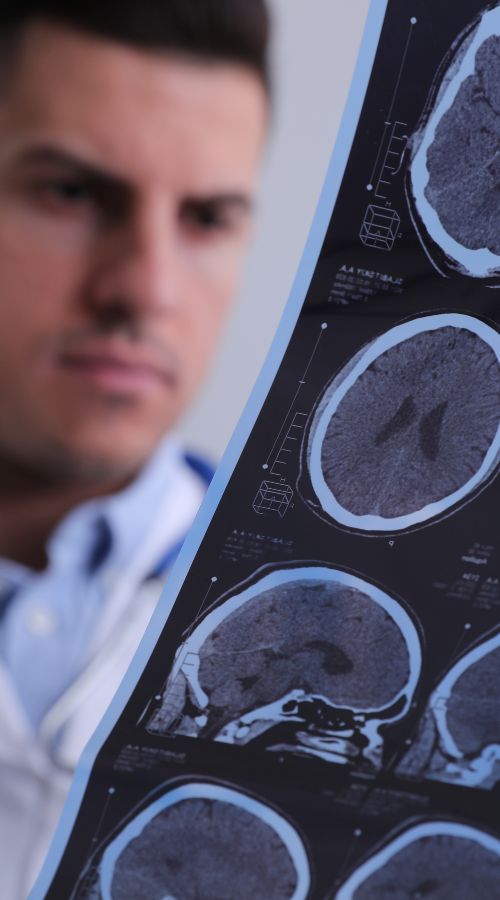
Epidiolex is a highly purified, pharmaceutical-grade CBD medication designed specifically for treating seizures associated with Lennox-Gastaut syndrome (LGS), Dravet syndrome, and Tuberous Sclerosis Complex (TSC).
While the exact mechanism of action is still being studied, Epidiolex is believed to work by modulating neural activity in ways that help reduce seizure frequency. Unlike other cannabis-based compounds, Epidiolex does not activate CB1 or CB2 cannabinoid receptors and doesn’t contain THC, meaning it does not cause psychoactive effects.
Epidiolex received FDA approval after multiple randomized, double-blind, placebo-controlled trials confirmed its effectiveness in reducing seizures:
In a clinical trial, Epidiolex reduced convulsive seizures by up to 44%, compared to a 27% reduction in the placebo group.
Patients taking Epidiolex experienced a 41–44% reduction in drop seizures, compared to 14% with a placebo.
The FDA expanded Epidiolex’s approval in 2020 after studies showed that 48% of patients experienced a 50% or greater reduction in seizure frequency.
Epidiolex is a highly purified form of cannabidiol, ensuring that each dose is free from THC, contaminants, and unregulated additives.
Unlike general CBD oils, which can differ in potency from batch to batch, Epidiolex is produced under strict quality control, guaranteeing precise and standardized dosing.
Perhaps most importantly, Epidiolex has undergone extensive clinical trials, demonstrating both safety and effectiveness in treating specific seizure disorders, a level of validation that over-the-counter CBD products do not have.
This is where regulatory caution comes into play. While Epidiolex is FDA-approved, over-the-counter CBD oils for epilepsy have not been clinically proven or regulated for seizure treatment.
Clinical trials have examined various CBD products, including CBD oils, transdermal applications, and long-term oral use, to determine their potential to reduce seizure frequency and severity. Some studies suggest that CBD may help manage seizures in certain cases, particularly in drug-resistant epilepsy, but results remain mixed, and more research is needed to confirm its effectiveness.
Many CBD epilepsy products on the market vary widely in:
A 2020 study analyzed various commercial CBD products and found that over 70% were mislabeled in terms of actual CBD content, raising concerns about their safety and consistency.
Patients who rely on accurate dosing for seizure control should exercise extreme caution when considering non-FDA-approved products.
Even with FDA-approved Epidiolex, CBD is not without side effects and risks. Reported side effects include:
Additionally, CBD can interact with other anti-seizure medications, altering their effectiveness. Patients considering CBD for epilepsy should always consult a neurologist or epilepsy specialist before making any changes to their treatment plan.
Epidiolex has brought new hope when it comes to CBD and epilepsy treatment for specific, severe conditions, and we’re eagerly waiting on more scientifically backed treatments. Hopefully, Epidiolex’s success will encourage new research and clinical trials that will help treat epilepsy with CBD products.
Until that happens, Epidiolex remains the only FDA-approved CBD medication for epilepsy, and while broader research is ongoing, no other CBD oils or extracts can claim the same level of regulatory approval or proven effectiveness.
For those living with epilepsy, consulting a medical professional is essential before considering any CBD-based treatment. While the potential of CBD epilepsy treatments is exciting, ensuring safety and effectiveness should always come first.